Abstract
Background: The current standard of care for transplant eligible patients with multiple myeloma, usually involves induction treatment with dexamethasone, a proteasome inhibitor and/or lenalidomide followed by autologous stem cell transplantation (ASCT). The optimal pre-transplant regimen and the extent to which we should try to deepen the pre-transplant remission remains undetermined. We therefore reviewed 496 patients with multiple myeloma who received ASCT at our institution with the goal of assessing predictors of outcome.
Methods: 496 patient received 596 transplants between January 2005 and December 2015. The median age was 61 years (range 39 - 84), 58% were male and 26% had high risk features defined by cytogenetics, FISH and/or gene expression profiling (GEP). All patients received dexamethasone as part of initial myeloma therapy, 426 patients (86%) received a bortezomib-containing regimen, 347 patients (70%) received a lenalidomide-containing regimen, 302 patients (61%) received both bortezomib and lenalidomide, and 10 patients (2%) received a carfilzomib-containing regimen. A total of 25 patients did not receive lenalidomide, bortezomib or carfilzomib. The median time from diagnosis to transplantation was 7 months. The median follow-up time was 52.2 months (0.4 to 139.6). We analyzed the impact of the following on OS: age, disease type, stage and risk, pre-transplant dialysis, induction regimen, mobilization regimen, time to transplantation, transplantation regimen, CD34+ cell dose collected and administered, engraftment syndrome, response pre and post transplantation, and number of transplants.
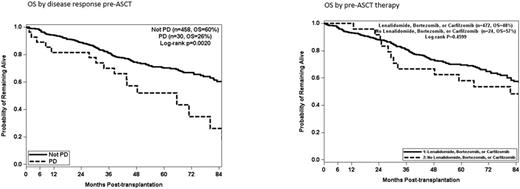
Results: We saw no difference in OS between patients receiving induction with lenalidomide, bortezomib or carfilzomib (either alone or in any combination) vs. no proteasome inhibitor or lenalidomide (p=0.4599). Having progressive disease at the time of transplantation predicted for a poorer OS (p=0.002) but all other responses (CR, VGPR, PR, SD) were not statistically different. These results apply to the entire cohort and individually for the low risk and high risk subsets. 11 patients had PD after first line therapy, of which only 1 was still in PD at the time of ASCT after receiving subsequent lines of therapy. However, of the 437 patients who were not in PD after first line therapy and continued treatment to deepen response, 29 patients evolved to PD at the time of transplantation. In multivariate analysis age > 60 (p=0.0059), high risk disease (p<0.0001), Mel 200 mg/m2 (p=0.0001) and PD response pre ASCT (p=0.0026) impacted OS.
Conclusion: There was no impact on OS by the use of lenalidomide, bortezomib or carfilzomib pre-transplantation. Response depth prior to transplantation, with the exception of patients with PD, had no impact on OS. In our cohort, 7% of patients who were in CR, VGPR, PR or SD after first line therapy and received further lines of therapy to deepen response, evolved to PD prior ASCT. Since PD is determined to be a predictor of adverse outcome, our data supports continuing to second-line therapy only for patients in PD after first line. All other patients should proceed to mobilization and ASCT.
McKiernan: Novartis: Speakers Bureau. Siegel: Celgene, Takeda, Amgen Inc, Novartis and BMS: Consultancy, Speakers Bureau; Merck: Consultancy. Vesole: Takeda: Speakers Bureau; Celgene: Speakers Bureau. Skarbnik: Genentech: Speakers Bureau; Seattle Genetics: Speakers Bureau; Novartis: Speakers Bureau; Gilead: Speakers Bureau; Abbvie: Other: Ad board, Speakers Bureau. Biran: Takeda: Speakers Bureau; Celgene, Amgen: Consultancy, Speakers Bureau. Richter: Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Speakers Bureau. Pecora: Caladrius Biosciences: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; COTA: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Goy: Genentech: Research Funding; Pharmacyclics / J&J: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Acerta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal